Public Health Agencies - RCKMS Decision Support & Authoring
Overview of RCKMS
The Reportable Conditions Knowledge Management System (RCKMS) is an authoritative, real-time portal to enhance disease surveillance. RCKMS provides comprehensive information on public health reporting requirements to public health reporters, such as clinicians and laboratories.
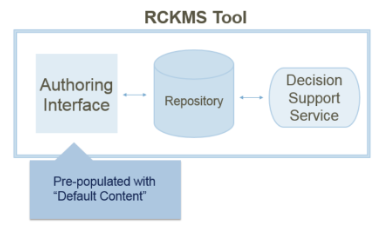
The RCKMS tool consists of three parts:
-
Authoring Interface: A web portal for public health agencies to input, edit, and manage their jurisdictional reporting criteria
-
Knowledge Repository: The database containing data on reporting specifications
-
Decision Support Service: A decision support service (DSS) that healthcare reporters can invoke to determine if a potential case is reportable, and to which jurisdiction(s)
How does RCKMS support eCR?
RCKMS is an integral piece of the electronic case reporting (eCR) architecture. Public health agencies (PHAs) populate the tool with jurisdiction reporting requirements so that electronic initial case reports (eICRs) from healthcare can be processed against them to determine if the case report is reportable and to which PHA(s) the report should be sent.
In order for eICRs to be fully processed in RCKMS, all PHA reporting specifications must be included. Currently, RCKMS is open for over 200 conditions and authoring is open to PHAs continuously. More information including the current list of conditions is available from the RCKMS website (external link). The condition codes for those conditions are available from the RCKMS Content Repository site (external link) and are used to map those SNOMED condition codes into public health surveillance systems.
More specifically, identified users from PHAs enter their jurisdictional reporting specifications through the RCKMS Authoring Interface:
-
To ease the burden of users having to input criteria from scratch, the authoring interface comes pre-populated with default reporting specifications developed from CSTE position statements.
-
The underlying rules of the criteria are run on a decision support service to determine reportability.
-
The criteria that can be automated are based on coded value sets containing SNOMED CT, ICD-10 CM, and LOINC codes for the given criteria to provide the automated matching capability.
-
-
Users can choose to adopt and use the default as is, or adjust it to meet their jurisdictional needs.
-
The tool also includes commonly requested optional reporting specifications jurisdictions can select.
-
If the listed options do not meet a jurisdiction’s needs, a user can submit a request to the RCKMS team for the addition of specific criteria.
After being entered by users, reporting specifications are stored in the knowledge repository and deployed to the decision support service. When an eICR is received on the Association of Public Health Laboratories (APHL) Informatics Messaging Services (AIMS) platform, the RCKMS DSS is invoked and a response is returned of whether the potential case report is reportable, to which jurisdiction(s) it may be reportable, and other relevant reporting information. As updates to reporting specifications are needed, a jurisdiction user can log into the RCKMS Authoring Interface to make the necessary changes and redeploy for those updates to take effect.
For more information about RCKMS, visit the RCKMS website (external link).
